The Company
Founded in 2002, the company quickly established itself as a leader in the generic drug market. Known for its commitment to innovation and continuous improvement, this company operates in over 20 countries and exports to over 150. With a strong focus on quality and continuous improvement, it offers high-quality medications at affordable prices, always centered on customer satisfaction and market leadership.
The Challenge
The company was facing a series of significant challenges that directly impacted its operational efficiency and ability to meet its production schedule.
Key issues identified included excessively high machine stoppage times in the packing area, frequent production schedule non-compliance, alarming levels of stockouts, and long setup times.
These problems were attributed to several root causes. The maintenance team handled machine setups, which should be ideally performed by operators. Challenges in managing tools and maintaining machinery regularly led to a decline in operational efficiency.
Additionally, failures in controlling the manufacturing process were apparent, worsened by the absence of structured and effective problem-solving. Finally, the operational standards for production, setups, and maintenance were ineffective in addressing current challenges, requiring review and improvement to restore and enhance productivity and efficiency.
The Approach
The company implemented a continuous improvement project with several key initiatives to address these challenges.
Machine setups were transferred to operators, increasing their autonomy and reducing reliance on the maintenance team. Setup standards were also established to reduce deviations and improve efficiency. Kobetsu Kaizen methodology was also used to reduce unplanned downtime and machine micro-failures.
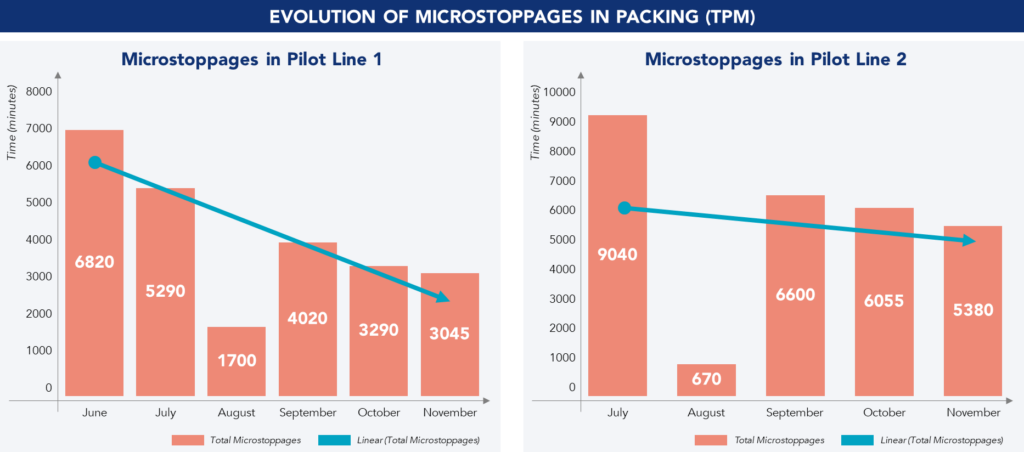
SMED was applied to train and develop operator skills, ensuring efficient process execution. 5S and visual management were implemented to improve tool accessibility and organization.

Alongside these initiatives, a Daily Kaizen program was created – a routine for team development and coordination through frequent meetings and data analysis for deviation response and decision-making.

Results
These initiatives led to significant improvements in the company’s performance indicators:
- 9.2% Increase in OEE: The overall equipment efficiency improved substantially.
- Greater Operator and Maintenance Autonomy: Empowering operators reduces dependence on the maintenance team.
- 37% Improvement in Setup Times: Standardization and responsibility transfer to operators resulted in faster and more efficient setups.
- 46% Reduction in Stockouts: Better tool and production process management led to fewer interruptions and product unavailability.
- 195% Improvement in Service Level: The ability to meet production and delivery deadlines increased significantly.
This company has established continuous improvement as a foundation of its organizational culture, creating a new paradigm where improvement is a daily commitment across all areas. This commitment to excellence has spread across all levels of the organization, involving all employees and fostering a dynamic work environment focused on constant innovation. This new context boosted productivity and enhanced the company’s adaptability to thrive in a competitive market.
See more on Improvement Projects
Find out more about improving this business area
See more on Pharmaceuticals
Find out more about transformation in this sector
